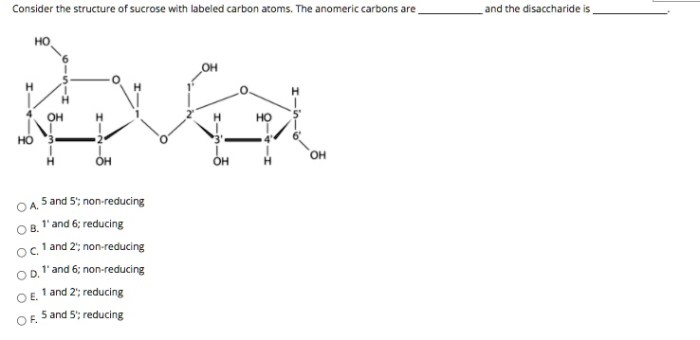
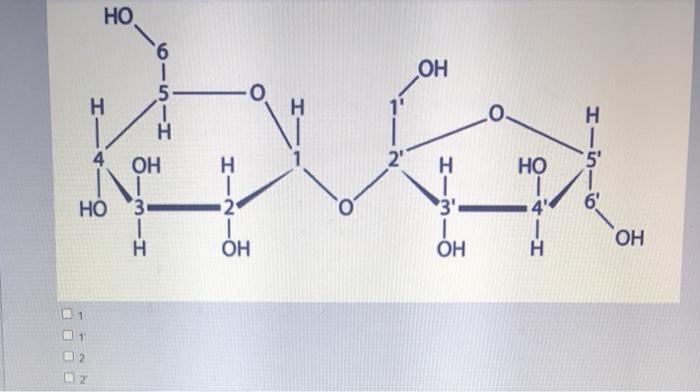
Consider the structure of sucrose with labeled carbon atoms, embarking on a scientific exploration that unveils the fundamental role of carbon atoms in shaping the identity and behavior of this ubiquitous carbohydrate. Sucrose, a disaccharide composed of glucose and fructose, holds immense significance in biological processes and industrial applications, and understanding its intricate structure is paramount to comprehending its diverse functions.
Delving into the molecular architecture of sucrose, we uncover a fascinating arrangement of carbon atoms, each occupying a distinct position and contributing to the molecule’s overall stability and reactivity. This detailed examination of carbon atoms’ placement and connectivity within the sucrose molecule provides a deeper understanding of its chemical and physical properties.
Structure of Sucrose
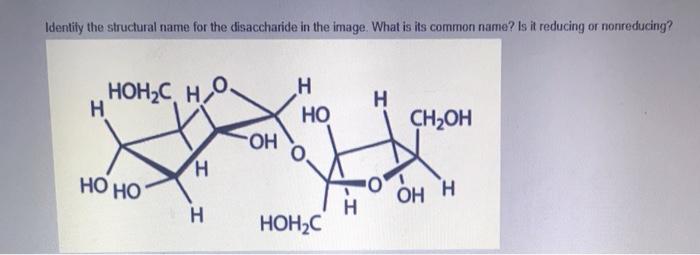
Sucrose, commonly known as table sugar, is a disaccharide composed of glucose and fructose units. It has the molecular formula C 12H 22O 11and a molecular weight of 342.30 g/mol.
The structure of sucrose consists of two monosaccharides, glucose, and fructose, linked together by a glycosidic bond between the C1 carbon of glucose and the C2 carbon of fructose.
Carbon Atoms in Sucrose, Consider the structure of sucrose with labeled carbon atoms
Sucrose contains 12 carbon atoms, labeled as C1 to C12. These carbon atoms are arranged in a specific sequence and connected by covalent bonds to form the disaccharide structure.
The carbon atoms in sucrose play a crucial role in determining its chemical and physical properties. The presence of multiple hydroxyl (-OH) groups and the glycosidic bond between the two monosaccharides influence the molecule’s solubility, reactivity, and other characteristics.
Chemical Properties of Sucrose
Sucrose is a non-reducing sugar, meaning it does not react with Benedict’s reagent or Tollens’ reagent. This is because the glycosidic bond between glucose and fructose forms a cyclic structure that prevents the formation of a free aldehyde or ketone group.
Sucrose undergoes hydrolysis in the presence of acids or enzymes to break down into its constituent monosaccharides, glucose, and fructose. This process is essential for the body to utilize sucrose as an energy source.
Physical Properties of Sucrose
Sucrose is a white, crystalline solid with a sweet taste. It is highly soluble in water and has a relatively high melting point of 186 °C.
The physical properties of sucrose are influenced by its molecular structure. The presence of multiple hydroxyl groups contributes to its water solubility, while the rigid ring structure of the disaccharide gives it a relatively high melting point.
Applications of Sucrose
Sucrose has a wide range of applications in food, pharmaceutical, and industrial sectors.
- Food industry:Sucrose is primarily used as a sweetener in various food products, including beverages, desserts, and baked goods.
- Pharmaceutical industry:Sucrose is used as an excipient in pharmaceutical formulations to improve the taste and stability of medications.
- Industrial applications:Sucrose is used in the production of biofuels, fermentation processes, and as a raw material for the synthesis of other chemicals.
Commonly Asked Questions: Consider The Structure Of Sucrose With Labeled Carbon Atoms
What is the molecular formula of sucrose?
C₁₂H₂₂O₁₁
How many carbon atoms are present in a sucrose molecule?
12
What is the significance of the glycosidic bond in sucrose?
The glycosidic bond links the glucose and fructose units, determining the stability and reactivity of the sucrose molecule.